The Process of Melting and Evaporation Involve Changes in What
Generally melting point increases with the increase in pressure. Melting change of a solid into a liquid when heat is applied.
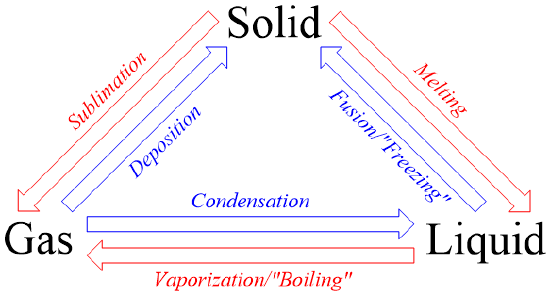
5 4 Phase Changes Chemistry Libretexts
In a solid the.

. Melting occurs when the molecules of a solid speed up enough that the motion overcomes the attractions so that the molecules can move past each other as a liquid. Describe the Process of Melting. How is thermal energy involved in the process of melting and evaporation.
Process by which a substance changes from a gas or vapor to a solid without first becoming a liquid. The surrounding gas must not be saturated with the substance which is evaporating. Therefore this process is said to involve a change in the state of matter of liquids.
The difference between evaporation and boiling point is that evaporation occurs only on the surface of a liquid while boiling occurs _____. It is also one of the three main steps in the global water cycle. These changes between states melting freezing and evaporating happen because as the temperature either increases or decreases the molecules in a substance begin to speed up or slow down.
Melting and evaporating are both a change in matteralso during evaporation and melting there is no change in temperature. The constant temperature at which the solid becomes liquid upon absorption of heat at constant pressure is called the melting point of that solid at that pressure. Amorphous non-crystalline substances such as glass or pitch melt by gradually decreasing in viscosity as.
The molecule s move and vibrate so quickly that they escape into the atmosphere as molecules of water vapor. When evaporation occurs the molecules left behind in the water _____. An impure solid generally melts over a range of temperatures below the melting point of the principal component.
If you move a substance from one container to another and its volume changes the substance is a a. When water is heated it evaporates. For example evaporation sublimation condensation or melting must involve heat transfer as an example The evaporation of water from a lake into A which is mess transfer requires a chance for latent heat of water.
Evaporation is a very important part of the water cycle. Electrolysis ____________ is the process of making a chemical reaction take place by passage of anelectric current through a substance or solution. Which of the following phase changes needs an increase of both temperature and kinetic energy.
Melting and freezing are _____. The process of melting and evaporation involve changes in the _____ of a substance. Students will see a small piece of ice melt on an aluminum surface.
Evaporation is a form of vaporisation that usually happens on the surface of liquids and it involves the transition of the liquid particles into the gaseous phase. The change of state from solid to gas without an intermediate liquid state is called _____ not evaporation. At a certain temperature the particles in a liquid have enough energy to become a gas.
The process of change of solid substance into its liquid state is called melting or fusion. Evaporation is the process by which a liquid turns into a gas. Plasma is hot highly ionized electrical conducting _____.
This is also true at the other four changes of phase. Newtons law of cooling applies to objects that _____. Freezing evaporation condensation and sublimation.
See answer 1 Best Answer. The process of thermal convection mainly involves _____. What process involves the change of state from solid to gas without passing the liquid state.
The process of a liquid becoming a gas is called boiling or vapourization while the process of a gas becoming a liquid is called condensation. This process is an example of a. Heat from the sun or solar energy powers the evaporation process.
In liquid water some particles have more energy. In a pure crystalline solid this process occurs at a fixed temperature called the melting point. The phase change between a liquid and a gas has some similarities to the phase change between a solid and a liquid.
Energy is either being used to break or form bonds and that is why the graph is flat at. Evaporation happens when a liquid substance becomes a gas. Cool or warm up.
The mass chancellor process does not have to involve heat transfer however a mass transfer process that involves a face change. Are less energetic result in lowered temperature have decreased average speeds. Melting is a process that causes a substance to change from a solid to a liquid.
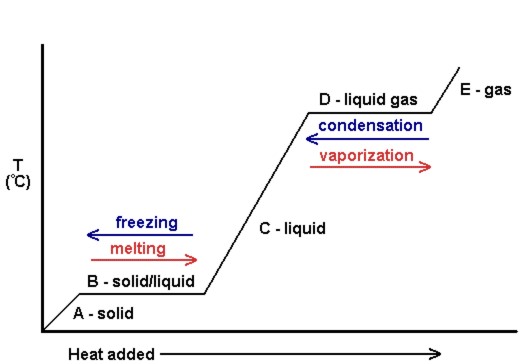
Phase Transitions Melting Boiling And Subliming Introductory Chemistry 1st Canadian Edition
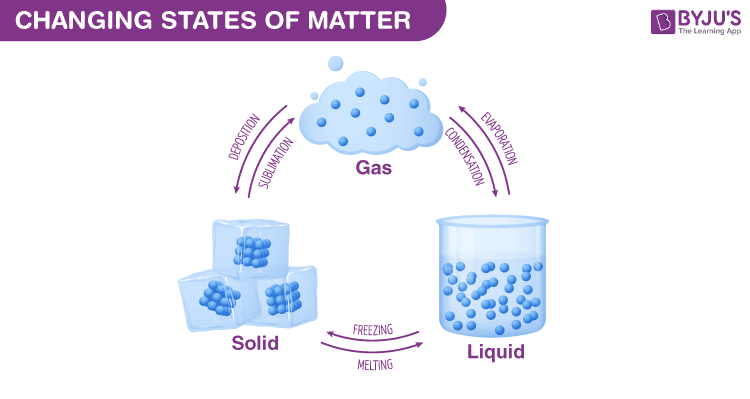
Changing States Of Matter Solid Liquid And Gas Phase Change

No comments for "The Process of Melting and Evaporation Involve Changes in What"
Post a Comment